
| Calcium | |
| Electrons per shell | 2,8,8,2 |
| Electron Configuration | [Ar] 4s2  |
| Ground state | 1S0 |
| Atomic Volume | 29.9 cm3 mol-1 |
| Electronegativity | 1.00 |
| Magnetic ordering | Paramagnetic |
| Mass magnetic susceptibility | 1.38 x 10-8 |
| Molar magnetic susceptibility | 5.531 x 10-10 |
| Speed of sound | 3810 m s-1 |
 Thermal Properties Thermal Properties |
|
| Enthalpy of Atomization | 184 kJ mol-1 @25°C |
| Enthalpy of Fusion | 9.33 kJ mol-1 |
| Enthalpy of Vaporisation | 150.6 kJ mol-1 |
| Heat Capacity | 25.929 J mol-1 K-1 |
| Thermal Conductivity | 201 W m-1 K-1 |
| Thermal Expansion | 22.3 μm m-1 K-1 |
 Hardness Hardness |
|
| Brinell hardness | 167 MPa |
| Mohs hardness | 1.75 |
 Elastic Properties Elastic Properties |
|
| Bulk modulus | 17 GPa |
| Poisson ratio | 0.31 |
| Shear modulus | 7.4 GPa |
| Young's modulus | 20 GPa |
 Electrical Properties Electrical Properties |
|
| Electrical resistivity | 3.4 x 10-8 Ω m |
| Electrical conductivity | 0.298 106/cm Ω |
 Chemical Properties Chemical Properties |
|
| Electrochemical Equivalent | 0.7477 g Ah-1 |
| Electron Work Function | 2.87 eV |
| Valence Electron Potential (-eV) | 29 |
| Ionization Potential | |
| 6.113 | |
| 11.871 | |
| 50.908 | |
| Incompatibilities | Water, oxidizers, acids, air, chlorine, chlorine tri-fluoride (ClF3), fluorine, oxygen, silicon, sulfur. |
 Flammability Flammability |
|
 Energy Levels Energy Levels |
|
| 3.69168 KeV | |
| 3.68809 KeV | |
| 4.0127 KeV | |
| 0.3413 KeV | |
| 0.3413 KeV | |
| 0.3449 KeV | |
| 0.306 KeV | |
| 0.302 KeV | |
 Atomic Radii Atomic Radii |
|
| 180 pm | |
| 194 pm | |
| 174 pm | |
| No data. | |
| 133 pm | |
| 197 pm | |
 Oxidation States Oxidation States |
|
| Ca+2 | |
 Ionisation Energies (kJ mol-1) Ionisation Energies (kJ mol-1)  |
|
| 589.7 | |
| 1145 | |
| 4910 | |
| 6474 | |
| 8144 | |
| 10496 | |
| 12320 | |
| 14207 | |
| 18191 | |
| 20385 | |
 Vapour Pressure Vapour Pressure |
||||||
| P (Pa) | 1 | 10 | 100 | 1K | 10K | 100K |
| T (K) | 864 | 956 | 1071 | 1227 | 1443 | 1755 |
 Crystal Structure Crystal Structure |
|
| Cubic close packed | |
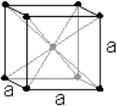 |
a = 558.84 pm |
| b = 558.84 pm | |
| c = 558.84 pm | |
| α = 90° | |
| β = 90° | |
| γ = 90° | |